In organic chemistry, peptide synthesis is the production of peptides, in which multiple amino acids are linked via peptide bonds. In contrast the biological process of producing long peptides (proteins) is usually referred to as protein biosynthesis.
The idea of linking amino acids to chain is over 100 years old but it took about 50 additional years to find solutions for occurring problems. Robert Bruce Merrifield pioneered the solid-phase peptide synthesis1. With SPPS it is possible to synthesize peptides with a length up to 50 amino-acids. The technology enables the synthesis of natural peptides which are difficult to express in bacteria, the incorporation of unnatural amino acids and to generate unique peptides to optimize a desired biological response or other result.
The invention of peptide synthesis led to the development of different application areas in which synthetic peptides are now used, including the development of epitope-specific antibodies against pathogenic proteins, the study of protein functions and the identification and characterization of proteins. Synthetic peptides enable research on important cell signaling enzymes like kinases and proteases, especially to understand enzyme-substrate interactions. Finally, synthetic peptides are used as standards and reagents in mass spectrometry (MS)-based applications. antibodies-online offers a variety of high quality blocking peptides and labeling peptides
Solid-Phase Peptide Synthesis SPPS
To start the SPPS the C-terminus of the first amino acid is coupled to an activated solid support, commonly chemically unreactive polystyrol. The resin acts as the C-terminal protecting group, the immobilized protein can be retained during a filtration process while liquid-phase reagents and by-products of synthesis are flushed away.
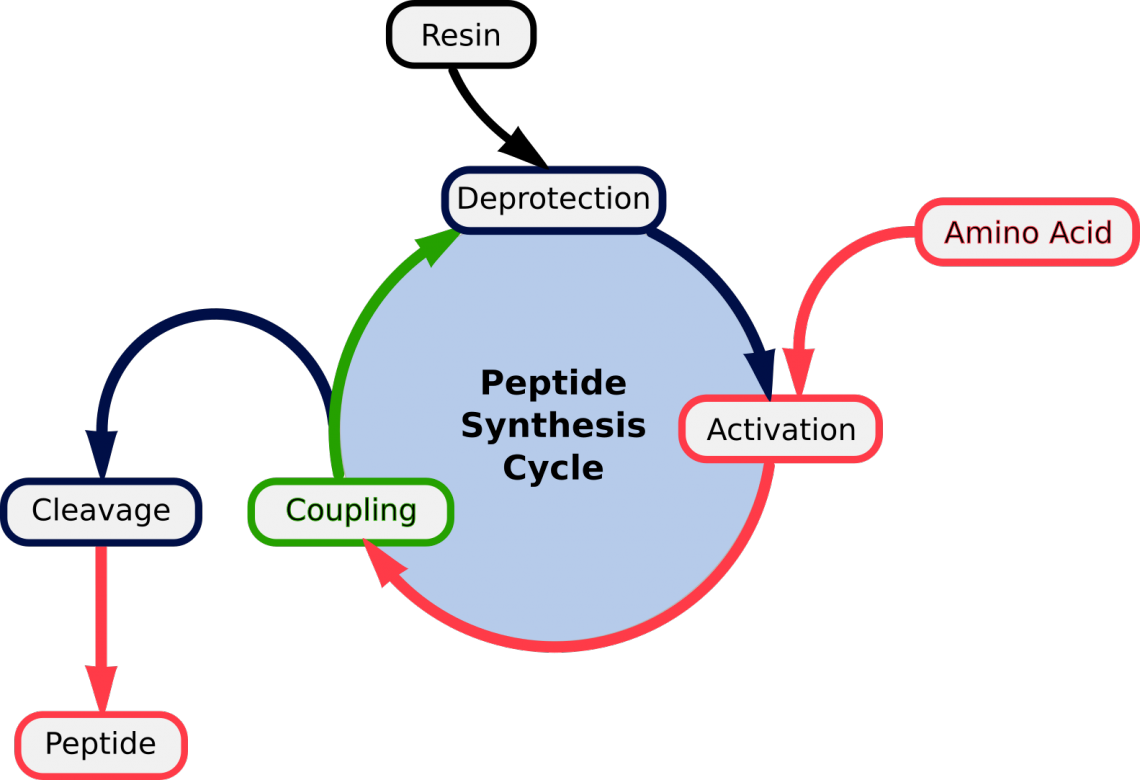
Figure 1. Repeated cycles of (1) Deprotection; (2) Activation of the aa; (3) Coupling | Detachment of mature peptide
The general principle of SPPS is one of repeated cycles of deprotection-wash-coupling-wash (fig.1). The free N-terminal amine of a solid-phase attached peptide is coupled (see below) to a single N-protected amino acid unit. This unit is then deprotected, revealing a new N-terminal amine to which a further amino acid may be attached.
Improvements and Variants
Over the years many variants and improvements of SPPS have been developed exchanging chemicals to optimize for a certain application2. Different resins allow for different functional groups at the C-terminus. The Wang resin and oxymethylphenylacetamidomethyl (PAM) resin results in the conventional C-terminal carboxylic acid3. On the other hand, the Rink amide and paramethylbenzhydrylamine (pMBHA) resin yields a C-terminal amide, which is useful in mimicking the interior of a protein. Fmoc (Fluorenyl-9-methoxycarbonyl) is an example for a base labile N α protecting group. Advantages are good acid stability, ultraviolet absorption which helps in monitoring and an easy preparation. Additionally side chains have to be protected as well to prevent the formation of branched chains. It should be permanent and compatible with Nα-protection however easy to remove with acid after completion of the synthesis.
Functional and Protecting Groups
| Functional group | Ser,Thr, Tyr | Asp, Glu | Lys | His | Cys |
| Protecting group | tert-Butylether | tert-Butylester | Boc | Boc / trityl | Trityl |
Figure 2. Functional and protecting groups in peptide synthesis.
The act of removing protecting groups, especially under acidic conditions, results in the production of cationic species that can alkylate the functional groups on the peptide chain (fig.2). Therefore, scavengers such as water, anisol or thiol derivatives can be added in excess during the deprotection step to react with any of these free reactive species.
Products
References:
- : "Solid-phase peptide synthesis and solid-phase fragment coupling mediated by isonitriles." in: Proceedings of the National Academy of Sciences of the United States of America, Vol. 110, Issue 29, pp. 11708-13, (2013) (PubMed).
- : "Guide for resin and linker selection in solid-phase peptide synthesis." in: Current protocols in protein science, Vol. Chapter 18, pp. Unit 18.7, (2008) (PubMed).
- : "p-alkoxybenzyl alcohol resin and p-alkoxybenzyloxycarbonylhydrazide resin for solid phase synthesis of protected peptide fragments." in: Journal of the American Chemical Society, Vol. 95, Issue 4, pp. 1328-33, (1973) (PubMed).

Creative mind of antibodies-online with a keen eye for details. Proficient in the field of life-science with a passion for plant biotechnology and clinical study design. Responsible for illustrated and written content at antibodies-online as well as supervision of the antibodies-online scholarship program.
Go to author page



