Ebola & Marburg Virus
Ebola virus disease (EVD) is a severe and frequently lethal disease caused by Ebola virus (EBOV). EVD outbreaks typically start from a single case of probable zoonotic transmission, followed by human-to-human transmission via direct contact or contact with infected bodily fluids or contaminated fomites. EVD has a high case–fatality rate; it is characterized by fever, gastrointestinal signs and multiple organ dysfunction syndrome. antibodies-online.com supports research on EBOV and MARV with a wide range of high-quality antibodies, proteins, lysates.
Ebola antibodies & proteins used in current research:
- (1)
- (1)
Ebola virus is one of the most virulent pathogens known to infect humans. The first recognized Ebola outbreak occurred in 1976, near the Ebola River in Zaire (now Democratic Republic of Congo, DRC). Over the past 40 years, more than 20 outbreaks have occurred in Africa, with most of the known outbreaks occurring in the past 20 years.
Marburgvirus (MARV) is closely related to the better-known Ebola virus and causes a similarly severe disease in humans. Some of the unique characteristics of filovirus outbreaks were reported for MARV disease (MVD) long before they were noticed in EBOV disease. A likely natural host for MARV and RAVV, and the probable source of the highly infectious viruses that cause Marburg virus disease (MVD) in humans, is the Egyptian fruit bat (Rousettus aegyptiacus).
Marburg virus and Ebola virus belong to the family Filoviridae. To date, 12 distinct filoviruses have been described. The seven filoviruses that have been found in humans belong either to the genus Ebolavirus (Bundibugyo virus (BDBV), Ebola virus (EBOV), Reston virus (RESTV), Sudan virus (SUDV) and Taï Forest virus (TAFV)) or to the genus Marburgvirus (Marburg virus (MARV) and Ravn virus (RAVV))(Fig 1). The WHO International Classification of Diseases Revision 11 of 2018 recognizes two major subcategories of filovirus disease (FVD): Ebola disease caused by BDBV, EBOV, SUDV or TAFV, and Marburg disease caused by MARV or RAVV.
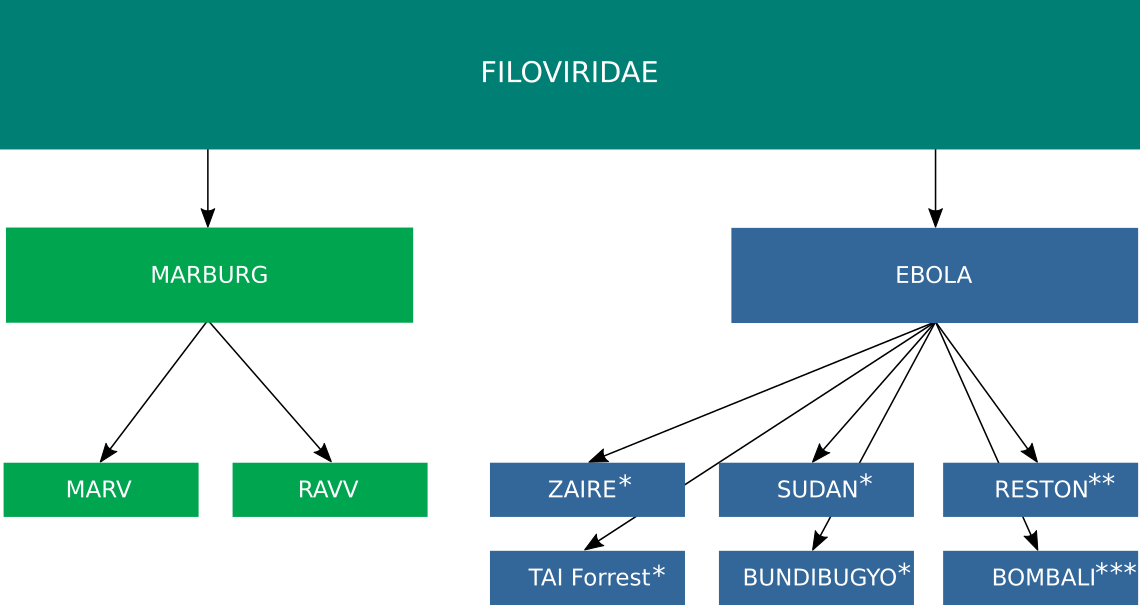
Fig. 1: Taxonomy of Ebola Virus. * Human infections associated with disease. ** Human infections not associated with disease. *** No human infections recorded.
Before 2014, almost all Ebola pipeline candidates were at the pre-clinical stage. Today, following a coordinated global effort, there are active pipelines for diagnostics, drugs and vaccines, with multiple candidates in late-stage clinical development. In 2018, the WHO approved five experimental therapies – three monoclonal antibodies (ZMapp, mAb114 and REGN-EB3) and two antiviral drugs (remdesivir and favipiravir) – for the treatment of Ebola under the MEURI protocol (adopted for the first time ever). The first-ever multi-drug trial investigating the efficacy of these drugs (other than favipiravir) began in 2018. In 2019, mAb114 and REGN-EB3 were deemed superior to ZMapp and both received Breakthrough Therapy designation from the FDA. Despite this success, there remains an unmet need for a therapeutic agent with pan-filovirus efficacy.
Genome Organization of Ebola Virus and Marburg Virus
The 19 kb viral genome encodes for seven main proteins: nucleoprotein (NP), glycoprotein (GP), L-polymerase (L) protein, viral protein (VP) 24, VP30, VP35, and VP40 (Fig 2). L is an RNA-dependent RNA polymerase (RdRp) and forms the RdRp complex with VP30 that is responsible for viral genome transcription and replication. VP24 and VP35 inhibit interferon signalling and facilitate evasion of the host immune response. NP encapsidates the viral genome into the nucleocapsid, whilst VP40 drives viral assembly and budding. GP is the only protein on the surface of the virion and is essential for binding to target cells and subsequently mediating membrane fusion and the release of the viral genome.
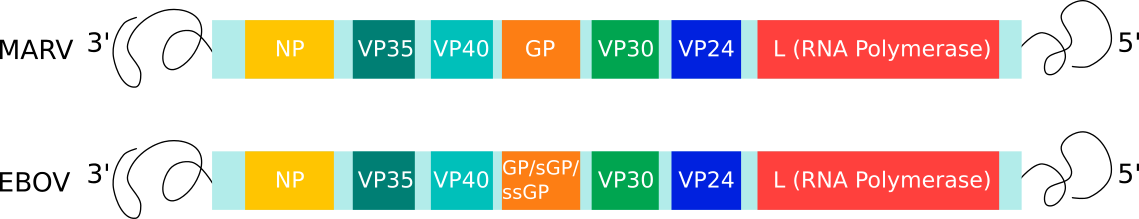
Fig. 2: Genome organisation of Filoviridae family members: MARV and EBOV. Each box represents the open-reading frames that produce the viral proteins.
Antibodies for Ebola & Marburg Virus Research
antibodies-online offers the following research antibodies for laboratory research on Ebola and Marburg virus. For more details click on the product links below. In case of any question do not hesitate to contact our scientific customer support.
Ebola Virus Glycoprotein Antibodies
Ebola Virus NP Antibodies
Ebola Virus VP24 Antibodies
Ebola Virus VP40 Antibodies
Marburg Virus Antibodies
Proteins for Ebola & Marburg Virus Research
antibodies-online offers the following proteins for laboratory research on Ebola and Marburg virus. For more details click on the product links below. In case of any question do not hesitate to contact our scientific customer support.
Ebola Virus Proteins
Bundibugyo Virus (BDBV) Proteins
Marburg Virus (MARV) Proteins
- (1)
Lysates for Laboratory Research
References
: "Ebolaviruses: New roles for old proteins." in: PLoS neglected tropical diseases, Vol. 12, Issue 5, pp. e0006349, (2018) (PubMed).: "Virus-encoded miRNAs in Ebola virus disease." in: Scientific reports, Vol. 8, Issue 1, pp. 6480, (2019) (PubMed).
: "A Comparison of the Pathogenesis of Marburg Virus Disease in Humans and Nonhuman Primates and Evaluation of the Suitability of These Animal Models for Predicting Clinical Efficacy under the 'Animal Rule'." in: Comparative medicine, Vol. 65, Issue 3, pp. 241-59, (2016) (PubMed).
: "Recent advances in marburgvirus research." in: F1000Research, Vol. 8, (2020) (PubMed).
: "Randomised controlled trial begins for Ebola therapeutics." in: Lancet (London, England), Vol. 392, Issue 10162, pp. 2338, (2019) (PubMed).
: "Ebola Virus Disease: Focus on Children." in: The Pediatric infectious disease journal, Vol. 34, Issue 8, pp. 893-7, (2016) (PubMed).
: "Ebola virus: A global public health menace: A narrative review." in: Journal of family medicine and primary care, Vol. 8, Issue 7, pp. 2189-2201, (2019) (PubMed).
: "Ebola virus disease." in: Nature reviews. Disease primers, Vol. 6, Issue 1, pp. 13, (2021) (PubMed).
- World Health Organization (WHO). Notes for the record: consultation on monitored emergency use of unregistered and investigational interventions for Ebola virus disease (EVD) [Internet]. 2018 May.
- LANDSCAPE OF EMERGING INFECTIOUS DISEASE RESEARCH AND DEVELOPMENT: PREVENTING THE NEXT PANDEMIC, G-Finder Project, 2020 September. Published by Policy Cures Research, Australia.

Master of Science in engineering. 12+ years of experience in marketing and e-commerce in the life science sector.
Go to author page



