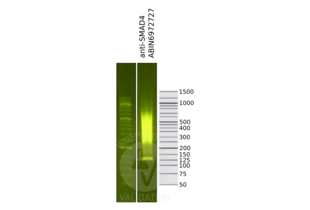
SMAD4 antibody (N-Term)
-
- Target See all SMAD4 Antibodies
- SMAD4 (SMAD Family Member 4 (SMAD4))
-
Binding Specificity
- N-Term
-
Reactivity
- Human
-
Host
- Rabbit
-
Clonality
- Polyclonal
-
Conjugate
- This SMAD4 antibody is un-conjugated
-
Application
- Western Blotting (WB), Immunoprecipitation (IP), Cleavage Under Targets and Release Using Nuclease (CUT&RUN)
- Characteristics
- SMAD4 (Mothers Against Decapentaplegic Homolog 4) or common SMAD (co-SMAD) is the coactivator and mediator of signal transduction by TGF-beta (transforming growth factor). Component of the heterotrimeric SMAD2/SMAD3-SMAD4 complex that forms in the nucleus and is required for the TGF-mediated signaling. Promotes binding of the SMAD2/SMAD4/FAST-1 complex to DNA and provides an activation function required for SMAD1 or SMAD2 to stimulate transcription. Component of the multimeric SMAD3/SMAD4/JUN/FOS complex which forms at the AP1 promoter site, required for syngernistic transcriptional activity in response to TGF-beta. May act as a tumor suppressor. Positively regulates PDPK1 kinase activity by stimulating its dissociation from the 14-3-3 protein YWHAQ which acts as a negative regulator. SMAD4 antibody (pAb) was raised in a Rabbit host. It has been validated for use in Immunoprecipitation and Western blot, it has been shown to react with Human samples.
- Purification
- Affinity Purified
- Immunogen
- This antibody was raised against a peptide within the N-terminal region of human SMAD4.
- Isotype
- IgG
-
-
- Application Notes
- Optimal working dilution should be determined by the investigator.
- Restrictions
- For Research Use only
-
- by
- Gianluca Zambanini, Anna Nordin and Claudio Cantù; Cantù Lab, Gene Regulation during Development and Disease, Linköping University
- No.
- #104457
- Date
- 12/07/2022
- Antigen
- SMAD4
- Lot Number
- 34614001
- Method validated
- Cleavage Under Targets and Release Using Nuclease
- Positive Control
Polyclonal rabbit anti-H3K4me (antibodies-online, ABIN3023251)
- Negative Control
Polyclonal guinea pig anti-rabbit IgG (antibodies-online, ABIN101961)
- Notes
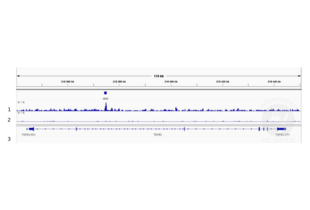
Passed. ABIN6972727 allows for CUT&RUN mediated profiling of SMAD4 in human cancer cells.
- Primary Antibody
- ABIN6972727
- Secondary Antibody
- Full Protocol
- Cell harvest and nuclear extraction
- Harvest 250,000 human cancer cells per antibody stimulated
- Centrifuge cell solution 5 min at 800 x g at RT.
- Remove the liquid carefully.
- Gently resuspend cells in 1 mL of Nuclear Extraction Buffer (20 mM HEPES-KOH pH 8.2, 20% Glycerol, 0.05% IGEPAL, 0.5 mM Spermidine, 10 mM KCl, Roche Complete Protease Inhibitor EDTA-free).
- Move the solution to a 2 mL centrifuge tube.
- Pellet the nuclei 800 x g for 5 min.
- Repeat the NE wash twice for a total of three washes.
- Resuspend the nuclei in 20 µL NE Buffer per sample.
- Concanavalin A beads preparation
- Prepare one 2 mL microcentrifuge tube.
- Gently resuspend the magnetic Concanavalin A Beads (antibodies-online, ABIN6952467).
- Pipette 20 µL Con A Beads slurry for each sample into the 2 mL microcentrifuge tube.
- Place the tube on a magnet stand until the fluid is clear. Remove the liquid carefully.
- Remove the microcentrifuge tube from the magnetic stand.
- Pipette 1 mL Binding Buffer (20 mM HEPES pH 7.5, 10 mM KCl, 1 mM CaCl2, 1 mM MnCl2) into the tube and resuspend ConA beads by gentle pipetting.
- Spin down the liquid from the lid with a quick pulse in a table-top centrifuge.
- Place the tubes on a magnet stand until the fluid is clear. Remove the liquid carefully.
- Remove the microcentrifuge tube from the magnetic stand.
- Repeat the wash twice for a total of three washes.
- Gently resuspend the ConA Beads in a volume of Binding Buffer corresponding to the original volume of bead slurry, i.e. 20 µL per sample.
- Nuclei immobilization – binding to Concanavalin A beads
- Carefully vortex the nuclei suspension and add 20 µL of the Con A beads in Binding Buffer to the cell suspension for each sample.
- Close tube tightly incubates 10 min at 4 °C.
- Put the 2 mL tube on the magnet stand and when the liquid is clear remove the supernatant.
- Resuspend the beads in 1 mL of EDTA Wash buffer (20 mM HEPES pH 7.5, 150 mM NaCl, 0.5 mM Spermidine, Roche Complete Protease Inhibitor EDTA-free, 2mM EDTA).
- Incubate 5 min at RT.
- Place the tube on the magnet stand and when the liquid is clear remove the supernatant.
- Resuspend the beads in 200 µl of Wash Buffer (20 mM HEPES pH 7.5, 150 mM NaCl, 0.5 mM Spermidine, Roche Complete Protease Inhibitor EDTA-free) per sample.
- Primary antibody binding
- Divide nuclei suspension into separate 200 µL PCR tubes, one for each antibody (150,000 cells per sample).
- Add 2 µL antibody (anti-SMAD4 antibody ABIN6972727, anti-H3K4me positive control antibody ABIN3023251, and guinea pig anti-rabbit IgG negative control antibody ABIN101961) to the respective tube, corresponding to a 1:100 dilution.
- Incubate at 4 °C ON.
- Place the tubes on a magnet stand until the fluid is clear. Remove the liquid carefully.
- Remove the microcentrifuge tubes from the magnetic stand.
- Wash with 200 µL of Wash Buffer using a multichannel pipette to accelerate the process.
- Repeat the wash five times for a total of six washes.
- pAG-MNase Binding
- Prepare a 1.5 mL microcentrifuge tube containing 100 µL of pAG mix per sample (100 µL of wash buffer + 58.5 µg pAG-MNase per sample).
- Place the PCR tubes with the sample on a magnet stand until the fluid is clear. Remove the liquid carefully.
- Remove tubes from the magnetic stand.
- Resuspend the beads in 100 µL of pAG-MNase premix.
- Incubate 30 min at 4 °C.
- Place the tubes on a magnet stand until the fluid is clear. Remove the liquid carefully.
- Remove the microcentrifuge tubes from the magnetic stand.
- Wash with 200 µL of Wash Buffer using a multichannel pipette to accelerate the process.
- Repeat the wash five times for a total of six washes.
- Resuspend in 100 µL of Wash Buffer.
- MNase digestion and release of pAG-MNase-antibody-chromatin complexes
- Place PCR tubes on ice and allow to chill.
- Prepare a 1.5 mL microcentrifuge tube with 102 µl of 2 mM CaCl2 mix per sample (100 µl Wash Buffer + 2 µL 100 mM CaCl2) and let it chill on ice.
- Always in ice, place the samples on the magnetic rack and when the liquid is clear remove the supernatant.
- Resuspend the samples in 100 µl of the 2 mM CaCl2 mix and incubate in ice for exactly 30 min.
- Place the sample on the magnet stand and when the liquid is clear remove the supernatant.
- Resuspend the sample in 50 µl of 1x Urea STOP Buffer (8.5 M Urea, 100 mM NaCl, 2 mM EGTA, 2 mM EDTA, 0.5% IGEPAL).
- Incubate the samples 1h at 4°C.
- Transfer the supernatant containing the pAG-MNase-bound digested chromatin fragments to fresh 200 µl PCR tubes.
- DNA Clean up
- Take the Mag-Bind® TotalPure NGS beads (Omega Bio-Tek, M1378-01) from the storage and wait until they are at RT.
- Add 2x volume of beads to each sample (e.g. 100 µL of beads for 50 µL of sample).
- Incubate the beads and the sample for 15 min at RT.
- During incubation prepare fresh EtOH 80%.
- Place the PCR tubes on a magnet stand and when the liquid is clear remove the supernatant.
- Add 200 µl of fresh 80% EtOH to the sample without disturbing the beads (Important!!! Do NOT resuspend the beads or remove the tubes from the magnet stand or the sample will be lost).
- Incubate 30 sec at RT.
- Remove the EtOH from the sample.
- Repeat the wash with 80% EtOH.
- Resuspend the beads in 25 µL of 10 mM Tris-HCl pH 8.2.
- Incubate the sample for 2 min at RT.
- Repeat the 2x beads clean up as described before (this time with 50 µL of beads for each sample).
- Resuspend the beads + DNA in 20 µL of 10 mM Tris-HCl pH 8.2.
- Library preparation and sequencing
- Prepare Libraries using KAPA HyperPrep Kit using KAPA Dual-Indexed adapters according to protocol.
- Sequence samples on an Illumina NextSeq 500 sequencer, using a NextSeq 500/550 High Output Kit v2.5 (75 Cycles), 36 bp PE.
- Peak calling
- Trim reads using using bbTools bbduk (BBMap - Bushnell B. - sourceforge.net/projects/bbmap/) to remove adapters, artifacts and repeat sequences.
- Map aligned reads to the hg38 human genome using bowtie with options -m 1 -v 0 -I 0 -X 500.
- Use SAMtools to convert SAM files to BAM files and remove duplicates.
- Use BEDtools genomecov to produce Bedgraph files.
- Call peaks using SEACR with a 0.001 threshold and the option norm stringent.
The protocol is published in Zambanini, G. et al. A New CUT&RUN Low Volume-Urea (LoV-U) protocol uncovers Wnt/β-catenin tissue-specific genomic targets. Development (2022). PMID 36355069
- Experimental Notes
The protocol is published in Zambanini, G. et al. A New CUT&RUN Low Volume-Urea (LoV-U) protocol uncovers Wnt/β-catenin tissue-specific genomic targets. Development (2022). PMID 36355069
Validation #104457 (Cleavage Under Targets and Release Using Nuclease)![Successfully validated 'Independent Validation' Badge]()
![Successfully validated 'Independent Validation' Badge]() Validation ImagesFull Methods
Validation ImagesFull Methods -
- Buffer
- Purified IgG in PBS with 30 % glycerol and 0.035 % sodium azide.
- Preservative
- Sodium azide
- Precaution of Use
- This product contains Sodium azide: a POISONOUS AND HAZARDOUS SUBSTANCE which should be handled by trained staff only.
- Storage
- -20 °C
- Storage Comment
- Avoid repeated freeze/thaw cycles by aliquoting items into single-use fractions for storage at -20°C for up to 2 years. Keep all reagents on ice when not in storage.
-
- Target
- SMAD4 (SMAD Family Member 4 (SMAD4))
- Alternative Name
- SMAD4 (SMAD4 Products)
- Molecular Weight
- 72 kDa
- NCBI Accession
- NP_005350
- Pathways
- Cell Division Cycle, Chromatin Binding, Autophagy
-

 (1 validation)
(1 validation)



